Mole fraction is not very useful for experiments that involve quantitative reactions, but it is convenient for calculating the partial pressure of gases in mixtures, as we saw in Chapter 10. As you will learn in Section 13.5, mole fractions are also useful for calculating the vapor pressures of certain types of solutions. Molality is particularly useful for determining how properties such as the freezing or boiling point of a solution vary with solute concentration. Units of ppb or ppm are also used to express very low concentrations, such as those of residual impurities in foods or of pollutants in environmental studies. How do chemists decide which units of concentration to use for a particular application? For many applications this may not be a problem, but for precise work these errors can become important.
In contrast, mole fraction, molality, and mass percentage depend on only the masses of the solute and solvent, which are independent of temperature. Because solution volumes vary with temperature, molar concentrations will likewise vary. When expressed as molarity, the concentration of a solution with identical numbers of solute and solvent species will be different at different temperatures, due to the contraction/expansion of the solution.
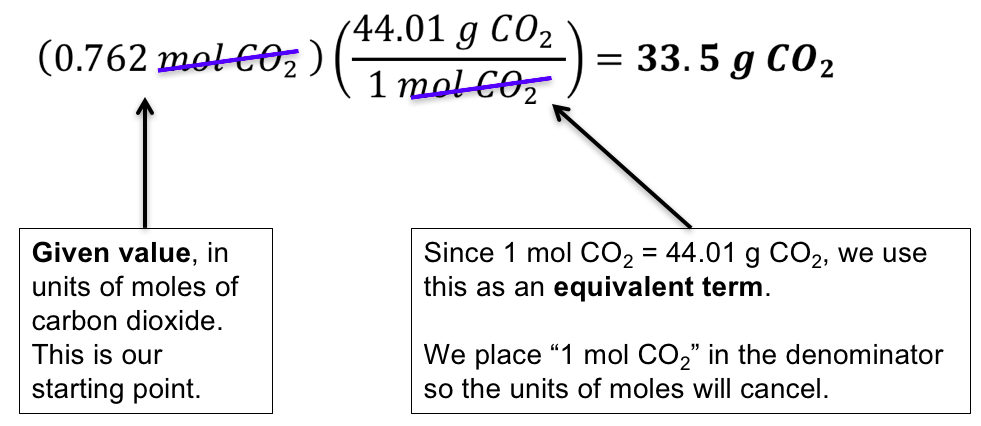
More appropriate for calculations involving many colligative properties are mole-based concentration units whose values are not dependent on temperature. Mole-mole calculations are not the only type of calculations that can be performed using balanced chemical equations. Recall that the molar mass can be determined from a chemical formula and used as a conversion factor.
For Equation 10.27 to be valid, the identity of the particles present cannot have an effect. Thus an ideal gas must be one whose properties are not affected by either the size of the particles or their intermolecular interactions because both will vary from one gas to another. The calculation of total and partial pressures for mixtures of gases is illustrated in Example 11.
There are several different ways to quantitatively describe the concentration of a solution. For example, molarity was introduced in Chapter 4 as a useful way to describe solution concentrations for reactions that are carried out in solution. Mole fractions, introduced in Chapter 10, are used not only to describe gas concentrations but also to determine the vapor pressures of mixtures of similar liquids. Example 4 reviews the methods for calculating the molarity and mole fraction of a solution when the masses of its components are known. The pressure exerted by each gas in a gas mixture is independent of the pressure exerted by all other gases present. Consequently, the total pressure exerted by a mixture of gases is the sum of the partial pressures of the components (Dalton's law of partial pressures).
The amount of gas present in a mixture may be described by its partial pressure or its mole fraction. The mole fraction of any component of a mixture is the ratio of the number of moles of that substance to the total number of moles of all substances present. In a mixture of gases, the partial pressure of each gas is the product of the total pressure and the mole fraction of that gas. Equilibrium constants which is expressed in terms of the mole fraction and partial pressure is symbolized as Kx, for both homogeneous and heterogeneous reactions involving gases. If we have a mixture of gases (A, B, C, etc.), then the mole fraction of gas A is worked out by dividing the number of moles of A by the total number of moles of gas.
This page explains equilibrium constants expressed in terms of partial pressures of gases, Kp. It covers an explanation of the terms mole fraction and partial pressure, and looks at Kp for both homogeneous and heterogeneous reactions involving gases. Finding the mole fraction of a particular constituent of a solution is useful for a number of reasons. Using the definition of a mole, it is possible to calculate several other figures based on the mole fraction.
Mole fractions are also used for many other things in chemistry, such as determining how much of a particular substance to dissolve into a solvent to obtain a solution of a particular concentration. Noting that the atomic mass of hydrogen is 1 amu and that of oxygen is 16 amu, the molar mass of acetone is 58 grams. Now you're ready to calculate mole fractions using the mole fraction equation.
The mole ratio is the fixed ratio between two species in a chemical reaction. It comes from the coefficients in front of the formulas in a balanced chemical equation.The mole ratio describes the fixed proportions between reactants and products in a chemical reaction. It is important in stoichiometry, particularly when used as a conversion factor in mole to gram conversions. Here is the mole ratio definition, with examples showing how to find the ratio and use it. Chemical reactions are always balanced using moles of the reactant and the product. The concentration of a solution involves the mole of a solute.
Some examples are molar concentration or molarity, molality, mole fraction, molar density. The mole fraction is another way of expressing the concentration. Concentration is an expression of how muchsoluteis dissolved in asolventin a chemicalsolution.
Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples. We have just calculated the partial pressures of the major gases in the air we inhale.
Experiments that measure the composition of the air we exhale yield different results, however. The following table gives the measured pressures of the major gases in both inhaled and exhaled air. Mole fraction and mass fraction are used to express the relative fractions of different constituents in a mixture. Both are unit-less terms since the ratios have the same unit, and thus the units cancel out. Mole fractions can be generated from various concentrations including molality, molarity and mass percent compositions. The free and best mole fraction calculator helps you to calculates the mole fraction, moles of solute, and moles of solvent according to the given inputs.
This mole fractions tool is quite easy to use which makes mole fraction calculation very simple and interesting. In percent composition problems, there are many possible solutions. For example, CH and C2H2 have the same proportions, but they are different compounds. It is standard to give compounds in their simplest form, where the ratio between the elements is as reduced as it can be-- called the empirical formula. When calculating the empirical formula from percent composition, one can convert the percentages to grams.
For example, it is usually the easiest to assume you have 100 g so 54.3% would become 54.3 g. Then we can convert the masses to moles; this gives us mole ratios. A good technique is to divide all the terms by the smallest number of moles.
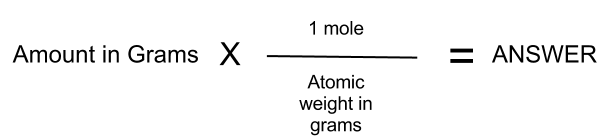
Then the ratio of the moles can be transferred to write the empirical formula. A mole is defined as the number of molecules present in 12 grams of pure carbon-12. This number, also known as Avogadro's number, is 6.02 x 1023.
A mole of any substance is contains this same number of molecules. This means that a mole of any substance has a mass of the combined atomic numbers of all the atoms present in one molecule of that material, in grams. This is useful in writing chemical equations and for other calculations in chemistry. Mole is the number of fundamental units of reactant substances involved in a chemical reaction whereas the fraction is referred to as the division of proportion of the reactant substances, respectively. Therefore, we can say that Mole Fraction equation is the ratio of the reactant substances that react/combine to form a product.
The mole ratios calculator is the best way to calculate mole fraction instantly. The mole fraction of any component of a mixture is referred to as the ratio of the number of moles of that substance to the total number of moles of all substances present. When it comes to the mixture of gases, the partial pressure of each gas is said to be as the product of the total pressure and the mole fraction of that gas. The concentration of a solution is the "strength" of a solution. A solution typically refers to the dissolving of some solid substance in a liquid, such as dissolving salt in water.
It is also often necessary to figure out how much water to add to a solution to change it to a specific concentration.The concentration of a solution is typically given in molarity. Molarity is defined as the number of moles of solute divided by the volume in liters of solution . Dissolving a nonvolatile substance in a volatile liquid results in a lowering of the liquid's vapor pressure. This phenomenon can be rationalized by considering the effect of added solute molecules on the liquid's vaporization and condensation processes. To vaporize, solvent molecules must be present at the surface of the solution.
The presence of solute decreases the surface area available to solvent molecules and thereby reduces the rate of solvent vaporization. While this interpretation is useful, it does not account for several important aspects of the colligative nature of vapor pressure lowering. A more rigorous explanation involves the property of entropy, a topic of discussion in a later text chapter on thermodynamics. A lower vapor pressure results, and a correspondingly higher boiling point as described in the next section of this module. Equilibrium constant for mole fraction is denoted by Kχ symbol.
Mole fraction and mass fraction are terms used to express the ratios between different components in compounds. Mole fraction can be converted into the mass fraction of the same compound and vice versa. One major benefit of the behavior of gases is that the volume of one ideal gas in a mixture of ideal gases is equivalent to its mole fraction. For all practical purposes, the volume fractions and the mole fractions of the components of an ideal gas mixture are interchangeable. It should be a trivial task now to extend the calculations to mass-mass calculations, in which we start with a mass of some substance and end with the mass of another substance in the chemical reaction.
For this type of calculation, the molar masses of two different substances must be used—be sure to keep track of which is which. Again, however, it is important to emphasize that before the balanced chemical reaction is used, the mass quantity must first be converted to moles. Then the coefficients of the balanced chemical reaction can be used to convert to moles of another substance, which can then be converted to a mass.
Mole fractionor molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. Note that moles cancel out when calculating mole fraction, so it is a unitless value. When this is done, the mole fraction is multiplied by 100%.
Reactants take a part in the chemical reaction and reactants are the substances which are combined with each other to make new substance or products. Reactants has its own impact on the chemical reaction therefore it becomes important to calculate the actual value of reactants. The mole fraction to mass fraction calculator can be very useful in this context.
How To Find Mole Fractions Chemistry Mole fraction is a measure of concentration of a chemical solution. It can be calculated by dividing the number of moles of one component of a solution by the total number of moles of all components of the solution. The sum of mole fractions of all components in the solution is always equal to 1. When analyzing solutions, chemists measure concentrations of components in moles.
The mole fraction of a solute is the ratio of the number of moles of that solute to the total number of moles of solute and solvent in solution. Because it's a ratio of moles to moles, the mole fraction is a dimensionless number, and of course, it's always less than one. In electrolyte solutions it is common to distinguish between the solvent and the dissolved substance, or solute, which dissociates into ions. For these solutions it is useful to express composition in terms of molality, designated as m, a unit proportional to the number of undissociated solute molecules per 1,000 grams of solvent.
The number of molecules or ions in 1,000 grams of solvent usually is very large, so molality is defined as the number of moles per 1,000 grams of solvent. Solutions whose components have significantly different vapor pressures may be separated by a selective vaporization process known as distillation. Consider the simple case of a mixture of two volatile liquids, A and B, with A being the more volatile liquid. By appropriately heating the mixture, component A may be vaporized, condensed, and collected—effectively separating it from component B.
The mole fraction calculator is the online tool that allows you to measure mole fraction, moles of solute, and even the moles of solvent corresponding to the given chemical solution parameters. Yes, calculating mole fraction becomes easy with this handy calculator. All you need to add a few parameters in the above moles of solute calculator to get mole fraction. The mass fractions of all components is equal to 1 since the mass fraction is a ratio. The mass fractions of individual components are always lower values than 1. In elemental analysis calculations, mass fraction refers to the ratio between the mass of a chemical element and the compound.
The mass fraction is independent of temperature because mass does not change when the temperature is changed. The mole fractions of all components equals 1 since the mole fraction is a ratio. The mole fraction can be used to express the mole percentage by multiplying the mole fraction from 100.
The mole fraction can also be called the amount fraction because moles give the amount of a constituent. Mole fraction is unit-less since it is a ratio between moles . Kp has exactly the same format as Kc, except that partial pressures are used instead of concentrations. The gases on the right-hand side of the chemical equation are at the top of the expression, and those on the left at the bottom. In other words, the division of the number of moles of the solute with the number of moles of both solute and solvent is equal to the mole fraction of the respective solute in a chemical reaction or solution.
Using the mole ratios calculator is helpful while calculating it online. In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to total pressure of the mixture. To calculate mole fraction of solute you first calculate the moles of the solute and then you divide that by the total number of moles of solute and solvent. The definition of a mole is thus the mass of any compound, measured in grams, that equals the masses of the component elements measured in atomic mass units.